Micro-CT Scans Reveal the Bladder Isn’t Just a Simple Balloon
:::info Authors:
(1) Fatemeh Azari, Department of Mechanical Engineering and Materials Science, University of Pittsburgh, 3700 O'Hara Street Benedum Hall of Engineering, Pittsburgh, PA 15261 ([email protected]);
(2) Anne M. Robertson, Department of Mechanical Engineering and Materials Science, University of Pittsburgh, 3700 O'Hara Street Benedum Hall of Engineering, Pittsburgh, PA 15261 and Department of Bioengineering, University of Pittsburgh, 3700 O'Hara Street Benedum Hall of Engineering, Pittsburgh, PA 15261 ([email protected]);
(3) Lori A. Birder, Department of Medicine, University of Pittsburgh, 3550 Terrace St, Pittsburgh, PA 15213 and Department of Pharmacology and Chemical Biology, University of Pittsburgh, 3550 Terrace St, Pittsburgh, PA 15213 ([email protected]).
:::
Table of Links
Summary and 1 Introduction
- Methodologies
- Results and Conclusions, and References
SUMMARY
Current mechanical models of the bladder largely idealize the bladder as spherical with uniform thickness. This present study aims to investigate this idealization using micro-CT to generate 3D reconstructed models of rat bladders at 10-20 micrometer resolution in both voided and filled states. Applied to three rat bladders, this approach identifies-shape, volume, and thickness variations under different pressures. These results demonstrate the filling/voiding process is far from the idealized spherical inflation/contraction. However, the geometry idealizations may be reasonable in cases where the filled bladder geometry is of importance, such as in studies of growth and remodeling.
1 INTRODUCTION
The bladder's functionality is determined by its geometry, wall thickness, and biomechanical properties, all susceptible to impairment due to aging and disease, exemplified by BOO. The importance of BOO for the population is highlighted by the globally escalating prevalence of benign prostatic hyperplasia (BPH), the central contributor to BOO. BPH has shown a pronounced increase of 70.5% from 51.1 million cases in 2000 to 94 million in 2019 [1,2,3] and is a particularly important medical problem for men aged 50-60 years from lower socio-economic backgrounds. BPH induces a spectrum of urinary dysfunctions [4-5] including bladder wall (BW) hypertrophy, changes in bladder dynamics, trabeculation, diverticula, hematuria, and the formation of bladder stones, all of which severely affect bladder compliance during filling and voiding. This complex scenario underscores the necessity for comprehensive research into the BW's evolving properties during BOO progression so that both pharmacological and surgical interventions for BPH can be improved.
\ Traditionally, the imaging modality of choice for BOO patients has been ultrasound; however, it fails to offer comprehensive insights into wall changes arising from tissue growth and remodeling. Since 1991, there has been intermittent exploration into the geometrical properties of the BW during the filling and voiding cycles. Nonetheless, this field of inquiry is hindered by the paucity of integrated interdisciplinary methodologies and the requisite sophisticated equipment [4,5,6]. Existing scholarly literature delineates two primary experimental approaches for the elucidation of BW properties: analyses conducted at the organ level and those at the tissue strip level [7,8,9]. While investigations at the tissue level, such as uniaxial and biaxial testing, facilitate a detailed examination of layer- specific properties, associated studies of whole bladder structure and function are needed for understanding whole-organ function [10,11,13].
\ Moreover, conventional approaches to bladder mechanics characterization have historically adopted an oversimplified model of the bladder, conceptualizing it as a uniformly thick, spherical vessel. While these idealizations may be appropriate in some settings, they fail to account for the organ’s complex geometry including the changing and non-uniform wall thickness during the filling process. More sophisticated models of the bladder are needed to authentically reproduce the bladder's complex biomechanical behavior. In response to this gap, our investigation employed micro-CT to precisely quantify the geometric properties of the bladder wall within an ex-vivo filling model.
2 METHODOLOGIES
2.1 Ex-Vivo Filling of the Urinary Bladder Organ
The urinary bladder, ureters, and urethra were surgically removed from three 3-4-month-old female Sprague-Dawley rats and immediately placed in a HEPES-buffered physiological saline solution (HB-PBS) with a composition of 134 mM NaCl, 6 mM KCl, 1 mM MgCl2, 2 mM 𝑀𝑔𝐶𝑙2, 10 mM HEPES, and 7 mM glucose, adjusted to a pH of 7.4. Calcium channel blockers were added to the solution to prevent spontaneous contraction of smooth muscle cells (SMC). The ureters were cauterized adjacent to the bladder wall and the urethra tied with 3-0 sutures to a 26G needle. All surrounding connective tissue was excised prior to mounting the bladder in the experimental apparatus. The urethra was cannulated and connected to the syringe pump (BS-8000, Braintree SCIENTIFIC INC) without pre-conditioning, delivering air into the bladder at a syringe translation rate of 1.5 ml/min until reaching a specified transmural pressure of 50-80 mmHg. Transmural pressure was quantitatively measured using a pressure transducer (PX409-OMEGA ENGINEERING INC.) placed near the bladder within the flow circuit. After achieving the target pressure, filling ceased, the valve was closed and the bladder was removed from the apparatus in preparation for micro-CT scanning.
2.2 Micro-CT Experimental Design and Morphological Analysis
he central steps to obtain a 3D reconstructed model of each bladder are i) mounting, alignment, and scanning using a high-resolution Skyscan 1272 scanner (Bruker Micro-CT, Kontich, Belgium), ii) 3D reconstruction of the micro-CT Z stacks of 2D images utilizing Nrecon software (Bruker MicroCT, Kontich, Belgium), iii) morphological analysis of the 3D model using Simpleware ScanIP software (Synopsys, Sunnyvale, California), iv), segmentation of the internal (lumen) and external (ablumen) geometries in Meshmixer software (Autodesk, San Francisco, California), and thickness analysis using Materialize 3-matic software (Materialize GmbH, Munich, Germany). Briefly, the excised bladder was first positioned within a custom-designed holder, ensuring immobilization to prevent data artifacts during the scanning process. After sealing the Luer-lock adapter with parafilm, the holder was mounted in the micro-CT system.
\ ![Figure 1: Micro-CT visualization of bladder's internal structure (left) [13], highlighting urethra, trigone, mid-bladder, and dome regions (right)](https://cdn.hackernoon.com/images/fWZa4tUiBGemnqQfBGgCPf9594N2-xk8335d.png)
\ Micro-CT scans were performed using 80 kV source voltage and 125 𝜇𝐴 source current. Images were captured at a 10.8 𝜇𝑚 pixel size using a rotation step of 0.6 degrees, a 2048 x 2048 frame size without filtering, and an exposure time of 400 ms. The reconstruction of these images with NRecon software involved smoothing at level 1, addressing ring artifacts at 50%, and correcting for 2% beam hardening. Scanning time for bladders was kept less than 10 minutes to avoid dehydration. The segmentation process began by thresholding grayscale values to create masks, transforming the reconstructed 2D images into 3D models. These models were then converted into surface models to produce stereolithography (STL) data. The finalized STL files were analyzed in Materialize 3-matics, using the midplane thickness tool to assess wall thickness. Our analysis provided key statistics such as median, average, and standard deviation of wall thickness provided as the output of thickness analysis from 3-Matics. Additionally, a histogram with an adjustable range was generated, ensuring no data points were overlooked. Morphology was compared between voided and filled states with transmural pressures for the filled state of P=50 mmHg, 57 mmHg and 80 mmHg for bladders A, B, C, respectively.
3 RESULTS AND CONCLUSIONS
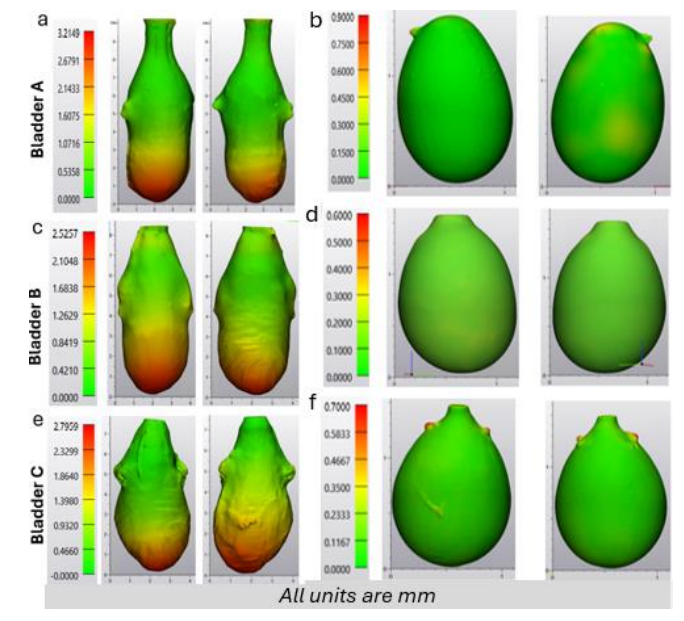
A quantitative assessment of bladder geometry was obtained for three rat bladder specimens, labeled as Bladder A, B, and C, Figure 2. Data was obtained at two inflation states: (1) voided (non-distended state – harvested condition) and (2) filled (distended). Bladder specific morphology results are given in Table 1.
\ 
\ 
\ 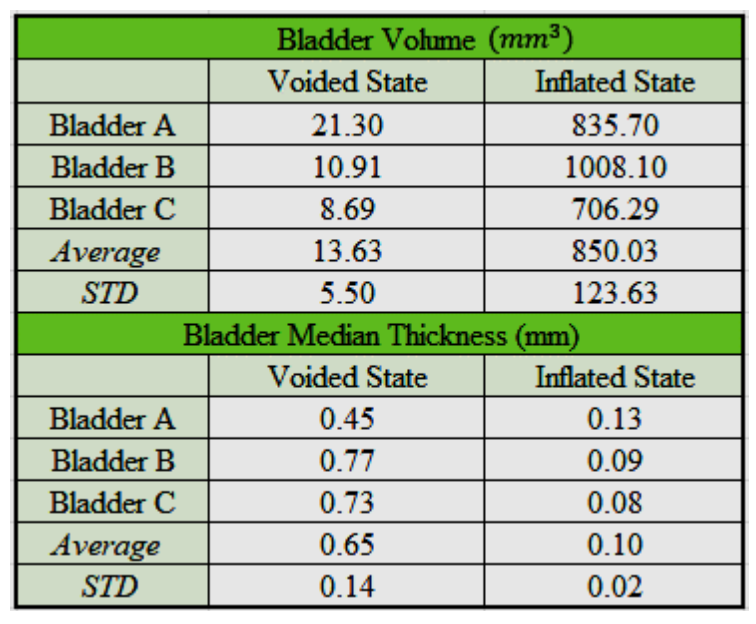
\ In the voided state, the wall thickness was highly non-uniform. For all three cases, the BW for the voided state was markedly thicker at the dome compared to the mid-bladder and trigonal areas. In particular, the average maximum dome thickness for all three bladders was 2.84 mm ±0.28 mm while the median thickness of the middle and trigonal areas was 0.65±0.14,respectively. In contrast, upon distension (Fig. 2, b, d, f), all three bladders have a relatively uniform, thin wall with an average median thickness of 0.10mm ±0.02mm.
\ Numerous models, including our own [3], have idealized the bladder with a spherical configuration and homogenous wall thickness [3,6,10], an approximation advantageous for deriving analytical solutions. The current work suggests this may be a reasonable approximation in studies where the filled bladder geometry is of importance. For example, in a recent study of growth and remodeling for BOO bladders, the filled (spherical) bladder was used as the reference configuration for defining the homeostatic stretch of both collagen fibers and smooth muscle cells [3]. However, it is possible that even the full bladder will show deviations in shape and wall thickness from this idealization in pathological states or as a result of aging [13]. A comparison of the shape and wall thickness between the voided and filled states, Fig 2, demonstrates that even for the healthy bladder, the filling process varies spatially over the wall of the bladder and is not well represented by simple spherical inflation. It is anticipated that heterogeneities in wall thickness will be even more complex during disease and a result of aging [13]. Hence, the changing shape and wall thickness during micturition will need to be quantified in these cases.
\ In summary, this work highlights the necessity to employ sophisticated imaging modalities, such as high-resolution micro-CT, to reveal bladder morphology and identify region-specific alterations in wall thickness throughout the filling and voiding process. Such high-resolution data is vital for computational mechanics models of the bladder needed for studying evolving bladder functionality during diseases such as bladder outlet obstruction (BOO). The authors express their gratitude for the funding received from NIH-R01 AG056944 and NIH-R01 DK133434.
REFERENCES
[1] Awedew, A. F., et al. The global, regional, and national burden of benign prostatic hyperplasia in 204 countries and territories from 2000 to 2019: a systematic analysis for the Global Burden of Disease Study 2019. The Lancet Healthy Longevity, 3(11), e754-e776, 2022.
\ [2] Fusco, F., et al. Progressive bladder remodeling due to bladder outlet obstruction: a systematic review of morphological and molecular evidence in humans. BMC urology, 18, 1-11, 2018.
\ [3] Cheng, F., et al. A constrained mixture-micturition-growth (CMMG) model of the urinary bladder: Application to partial bladder outlet obstruction (BOO). Journal of the mechanical behavior of biomedical materials, 134, 105337, 2022.
\ [4] Damaser, M. S. et al. Partial outlet obstruction induces chronic distension and increased stiffness of rat urinary bladder. Neurourology and Urodynamics: Official Journal of the International Continence Society, 15(6), 650-665, 1996.
\ [5] Parekh, A., et al. Ex vivo deformations of the urinary bladder wall during whole bladder filling: contributions of extracellular matrix and smooth muscle. Journal of biomechanics, 43(9), 1708-1716, 2010.
\ [6] Trostorf, R., et al. A pilot study on an active and passive ex vivo characterization of the urinary bladder and its impact on three-dimensional modelling. journal of the mechanical behavior of biomedical materials, 133, 105347, 2022.
\ [7] Trostorf, R., et al. Location-and layer-dependent biomechanical and microstructural characterization of the porcine urinary bladder wall. Journal of the mechanical behavior of biomedical materials, 115, 104275, 2021.
\ [8] Hanczar, M., et al. The Significance of Biomechanics and Scaffold Structure for Bladder Tissue Engineering. International Journal of Molecular Sciences, 22(23), 12657, 2021.
\ [9] Ajalloueian, F., et al. Bladder biomechanics and the use of scaffolds for regenerative medicine in the urinary bladder. Nature Reviews Urology, 15(3), 155-174, 2018.
\ [10] Hennig, G., et al. Quantifying whole bladder biomechanics using the novel pentaplanar reflected image macroscopy system. Biomechanics and Modeling in Mechanobiology, 1- 11, 2023.
\ [11] Damaser, M. S. Whole bladder mechanics during filling. Scandinavian Journal of Urology and Nephrology, 33(201), 51-58, 1999
\ [12] “X-ray micro-ct analysis,” DigiM Solution, https://digimsolution.com/services/imaging/xray-micro-ct-analysis, 2023
\ [13] Birder, L. A., et al. Hypoxanthine Induces Signs of Bladder Aging with Voiding dysfunction and Lower Urinary Tract Remodeling. The Journals of Gerontology: Series A, glad171, 2023.
\
:::info This paper is available on arxiv under CC BY 4.0 DEED license.
:::
\
You May Also Like

Cardano Price Prediction: ADA To Rally 6000%? Win For Grayscale Large Cap Fund

Coinbase Integrates Crypto Trading with Samsung Pay for 75M Users
